Recently, Dr. NAN Jingbo from the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences, Dr. LUO Shunqin from Japan’s National Institute for Materials Science (NIMS), and Dr. Quoc Phuong Tran from the University of New South Wales, Australia, along with researchers from other institutions, published a new study in Nature Communications. Their research highlights the potential role of iron sulfides in catalyzing the reduction of gaseous carbon dioxide (CO₂) into prebiotic organic molecules through non-enzymatic pathways in early Earth’s terrestrial hot springs. This work offers new insights into Earth’s early carbon cycles and prebiotic chemical reactions, underscoring the significance of iron sulfides in supporting the terrestrial hot spring origin of life hypothesis.
Iron sulfides, abundant in early Earth’s hydrothermal systems, may have functioned similarly to cofactors in modern metabolic systems, potentially facilitating essential prebiotic chemical reactions. Previous studies on iron sulfides and the origin of life have focused primarily on deep-sea alkaline hydrothermal vents, where favorable conditions like high temperature, pressure, pH gradients, and hydrogen (H₂) from serpentinization were thought to support prebiotic carbon fixation. However, some scientists have proposed terrestrial hot springs as another plausible setting for life’s origins, as they contain rich mineral content, diverse chemicals, and abundant sunlight (Figure 1). To explore iron sulfides' role in terrestrial prebiotic carbon fixation, the research team synthesized a series of nanoscale iron sulfides (mackinawite) (Figure 2), including pure iron sulfide and iron sulfides doped with common hot spring elements including manganese, nickel, titanium, and cobalt. Experiments demonstrated that these iron sulfides could catalyze the H₂-driven reduction of CO₂ at specific temperatures (80–120°C) and atmospheric pressure, with gas chromatography quantifying the methanol produced (Figure 3).
The study found that manganese-doped iron sulfides exhibited notably high catalytic activity at 120°C. This activity was further enhanced under UV-visible (300–720 nm) and UV-enhanced (200–600 nm) light, suggesting that sunlight might play a role in driving this reaction by facilitating chemical processes. Additionally, the introduction of water vapor boosted catalytic activity, implying that vapor-laden terrestrial hot spring environments may have served as key sites for non-enzymatic organic synthesis on early Earth.
To further investigate the mechanism behind the H₂-driven CO₂ reduction, the team conducted in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analyses. Results indicated that the reaction likely proceeds via the reverse water-gas shift (RWGS) pathway, wherein CO₂ is first reduced to carbon monoxide (CO), which is then further hydrogenated to form methanol. Density functional theory (DFT) calculations provided additional insights, revealing that manganese doping not only lowered the reaction’s activation energy but also introduced highly efficient electron transfer sites, thereby enhancing reaction efficiency (Figure 4). The redox characteristics of iron sulfides make them functionally analogous to modern metabolic enzymes, providing a chemical foundation for prebiotic carbon fixation.
This research underscores the potential of iron sulfides in catalyzing prebiotic carbon fixation in early Earth’s terrestrial hot springs, opening new directions for exploring life’s origins and supporting future efforts in the search for extraterrestrial life.
Cite this article:
Nan, J., Luo, S., Tran, Q.P. et al. Iron sulfide-catalyzed gaseous CO2 reduction and prebiotic carbon fixation in terrestrial hot springs. Nat Commun 15, 10280 (2024). https://doi.org/10.1038/s41467-024-54062-y

Figure 1: Conceptual illustration of terrestrial hot springs on early Earth (Alex Bosoy Design)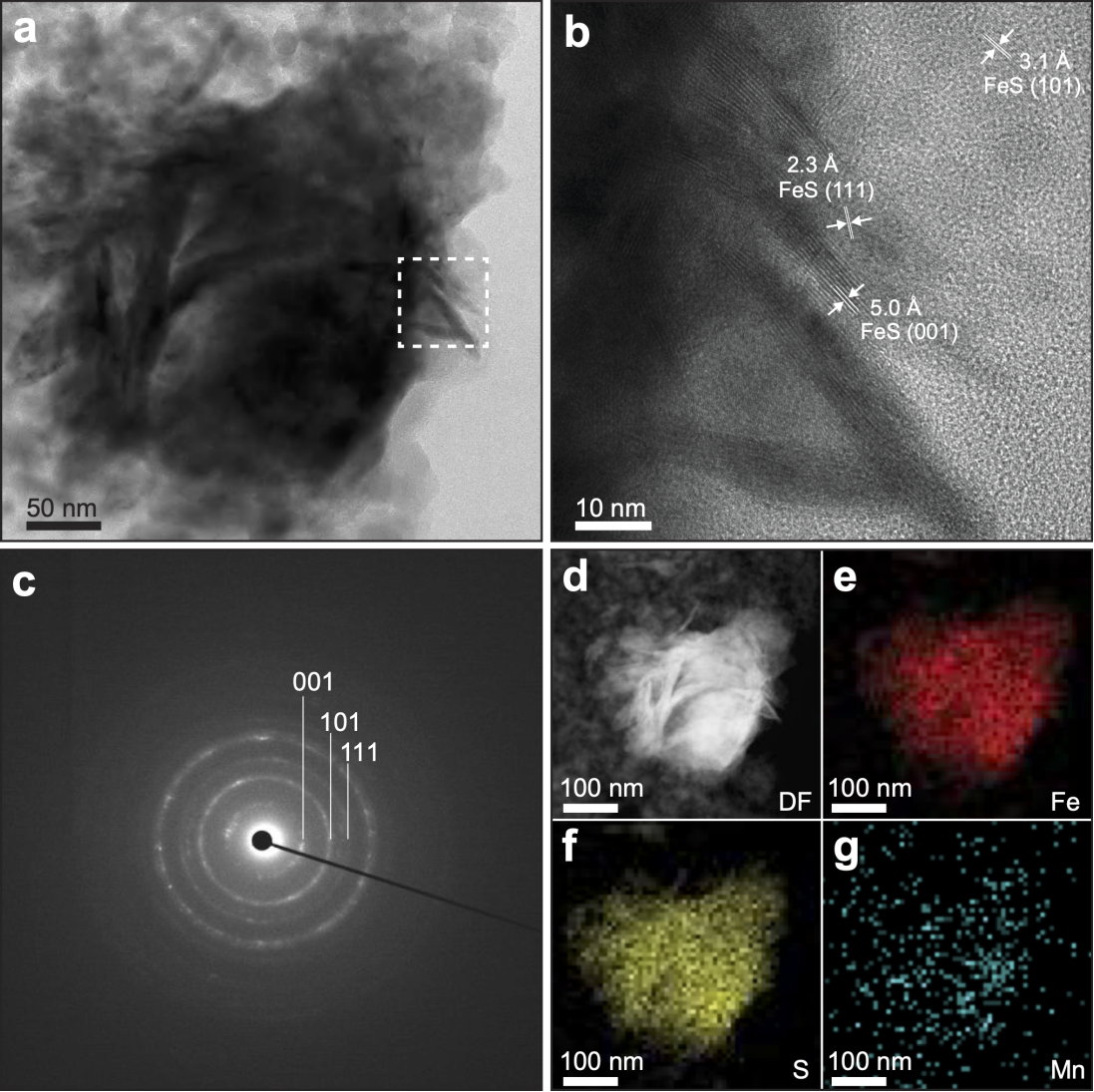
Figure 2: Scanning transmission electron microscopy reveals the characteristics of the iron sulfide (mackinawite) catalyst.
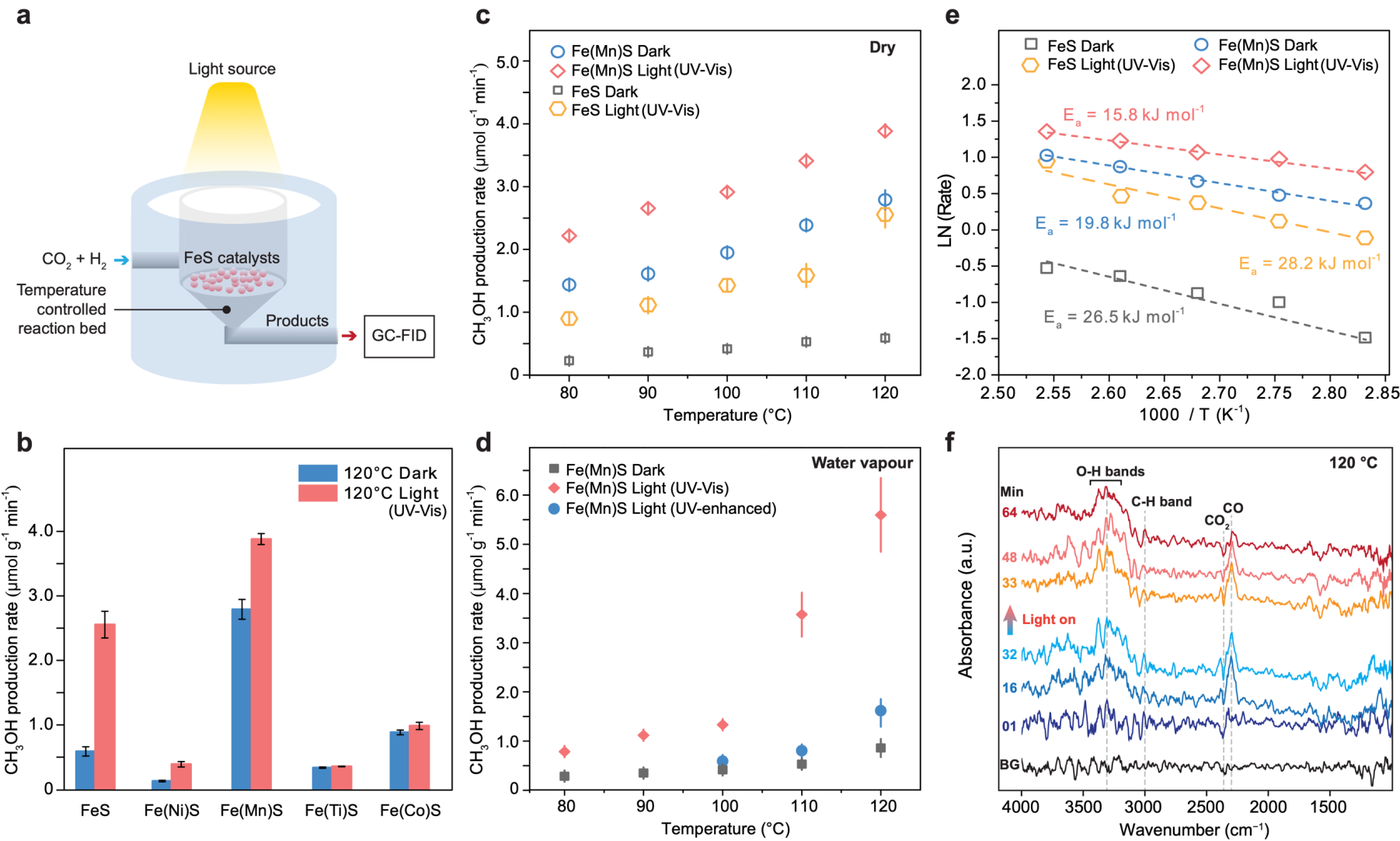
Figure 3: Simulated reaction of metal-doped iron sulfides catalyzing the H₂-driven reduction of CO₂ under various terrestrial hot spring conditions
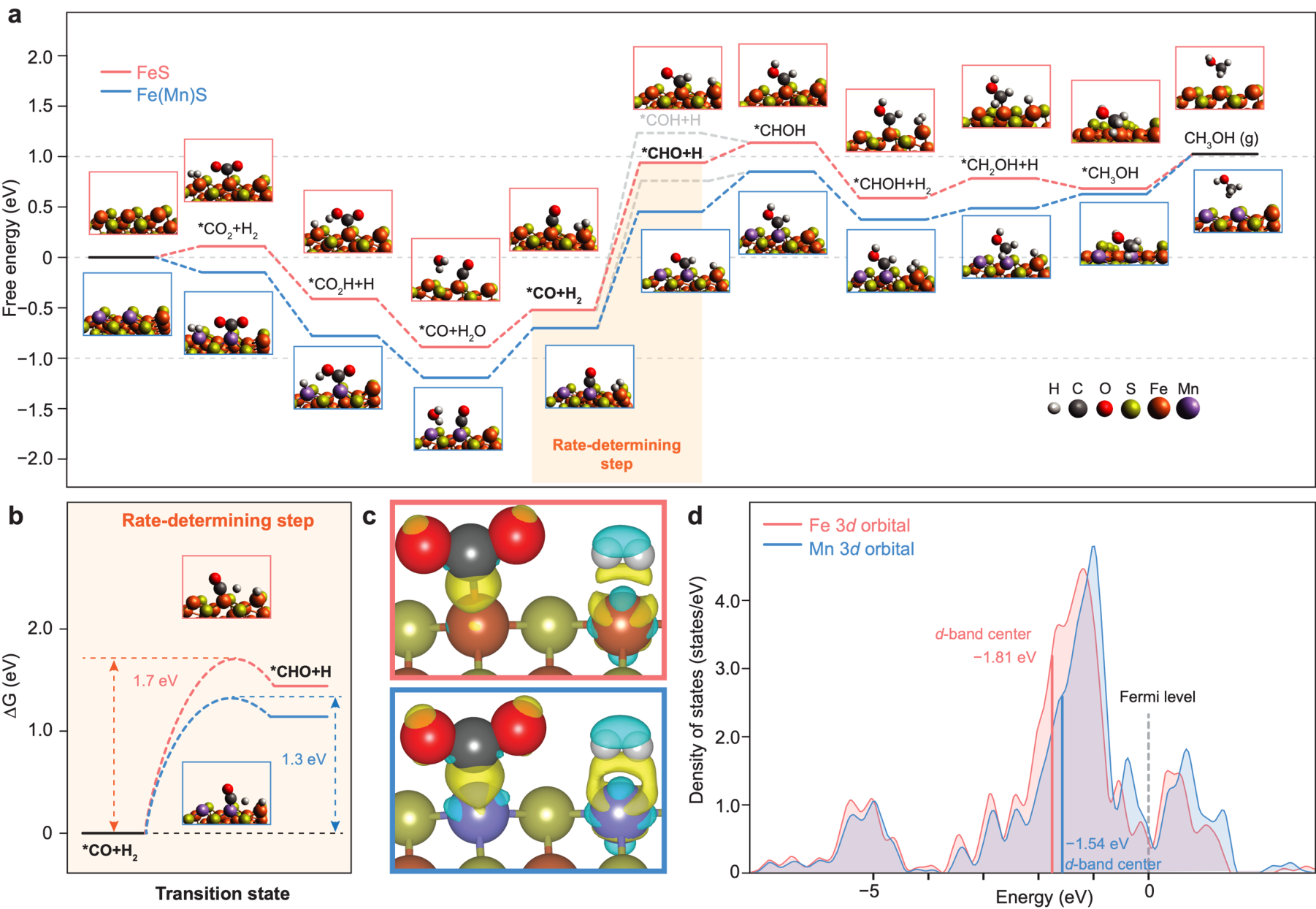
Figure 4: Density Functional Theory (DFT) calculations of CO₂ hydrogenation on the surfaces of pure iron sulfide and manganese-doped iron sulfide.
Download:
