Recently, Dr. JIANG Hui supervised by Professors WANG Bo and ZHANG Haichun from the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (NIGPAS), led a collaborative cicada study with scholars from several countries. Their research aimed to clarify the early evolutionary history of Cicadoidea fossils, the phylogenetic relationships between Mesozoic fossils and extant Cicadoidea, the macroevolution of body structure adaptations, and their relationship with environmental changes. The related findings were recently published in Nature Communications.
Cicada, here referring to the superfamily Cicadoidea, is well-known for its evolution of sound production system, exceptional long-term subterranean habits, and symbolic attributes and utility in research that widely exists in culture, life, and science. Extant Cicadoidea includes the globally widespread Cicadidae, commonly known as true/singing cicadas, and relictual Tettigarctidae, found only in Australia and colloquially known as hairy cicadas.
Currently, the earliest fossil of Cicadoidea was found in the Triassic. All Mesozoic (approximately 252 to 66 million years ago) Cicadoidea fossils have traditionally been classified into Cicadidae and Tettigarctidae based on a few distinct and conservative morphological features. However, this direct assignment of Mesozoic fossils to modern taxa may overlook the role of unique and transitional features provided by fossils in tracking their early evolutionary paths.
This study explores the phylogenetic relationships of fossil and extant Cicadoidea groups for the first time. It reveals that Mesozoic Cicadoidea fossils include stem cicadoids, stem tettigarctids, and stem cicadids. Some Mesozoic fossils previously classified as Tettigarctidae might actually be closer to modern Cicadidae phylogenetically. The clades of Cicadidae and Tettigarctidae might have diverged at or by the Middle Jurassic. Due to preservation issues, the classification of insect fossils often relies on the preserved partial morphological features.
This study conducted a morphological analysis of the partial structures of adults and nymphs, such as wings, non-wing body parts, and nymphal legs, to compare subtle continuous morphological changes with classification and phylogenetic outcomes. The research found that specialized homologous structures in insect fossils might contain previously overlooked identifiable transitional variations.
An in-depth examination of these continuous morphological changes can provide a more precise understanding of the impact of spatiotemporal changes on morphological evolution and further clarify patterns of macroevolution. For example, changes in the head and labium might reflect resistance adaptations due to feeding pressures from host plant changes. Additionally, changes in the thoracic notum, and alterations in wing venation and outline, may indicate the evolution of flight muscles and capabilities. Changes in the head and thorax can also be quantified and compared.
Producing sounds is a vital communication method for many animals. Modern Cicadidae species can produce the loudest sounds among insects, reaching nearly 120 decibels via tymbal mechanisms. In contrast, Tettigarctidae communicate with subtler vibrational signals transmitted through the substrate, lacking the ability to produce loud sounds. The varied sound-producing mechanisms between Cicadidae and Tettigarctidae ignite curiosity about the initial evolution of their acoustic structures and behaviours.
In this study, tymbals were identified in all Mesozoic cicadoid stem groups, preserved in both male and female specimens. This is the first identification of tymbal structures in Cicadoidea fossils, capturing this communication method in the fossil record. The majority of relatively intact fossils lacked elements for intricate sound production and auditory systems, suggesting mid-Cretaceous cicadoids may have relied on substrate-transmitted vibrations for communication, rather than producing or perceiving high-decibel songs.
Additionally, there are instances where the discovery of tymbal muscles and an abdominal cavity in a fossil, alongside preserved tracheae, flight muscles, and Marplesian tubes, suggests the possibility of an inherent abdominal cavity and resonating abilities similar to those found in the abdomens of modern singing cicadas. Consequently, the study posits another hypothesis: certain mid-Cretaceous Cicadoidea groups may have generated sounds louder than substrate-transmitted vibrations. Anyway, compared to modern singing cicadas, the species of Cicadoidea might have been relatively silent for the majority periods of Mesozoic.
This study also reports the oldest known Cicadoidea nymph and exuviae fossils from mid-Cretaceous Kachin amber. The prominently powerful fossorial forelegs of these nymphal fossils, akin to modern cicadas, suggest similar behaviours and robust capabilities for digging, soil transportation, and subterranean living. Cicada nymph and adult fossils show distinct ecological niches and survival strategies, with a notable shift from underground root feeding to above-ground stem feeding.
Evidence of root feeding is infrequently found in fossils. However, cicada nymph fossils with specialized digging forelegs suggest this behaviour. This subterranean lifestyle presumably conferred a survival benefit, enabling extended underground habitation for cicada nymphs.
This study also examines the occurrence of root feeding among arthropods in the fossil record. Given the adult and nymph Cicadoidea fossils from Kachin amber and the multitude of adult fossils from the Middle Jurassic Daohugou deposit, it's evident that mid-Mesozoic Cicadoidea demonstrated distinct life stage niches, facilitated biomass transfer from underground to above-ground, and influenced ecosystems in a manner akin to their modern cicadas.
This work is jointly funded by the National Natural Science Foundation of China and the Chinese Academy of Sciences.
Reference: Jiang Hui, Szwedo J., Labandeira C.C., Chen Jun, Moulds M.S., Mahler B., Muscente A.D., Zhuo De, Nyunt T.T., Zhang Haichun, Wei Cong, Rust J., Wang Bo (2024) Mesozoic evolution of cicadas and their origins of vocalization and root feeding. Nature Communications, 15: 376. https://doi.org/10.1038/s41467-023-44446-x.

Adults, final instar nymph, and exuviae of Cicadoidea fossils in Kachin amber of Myanmar.
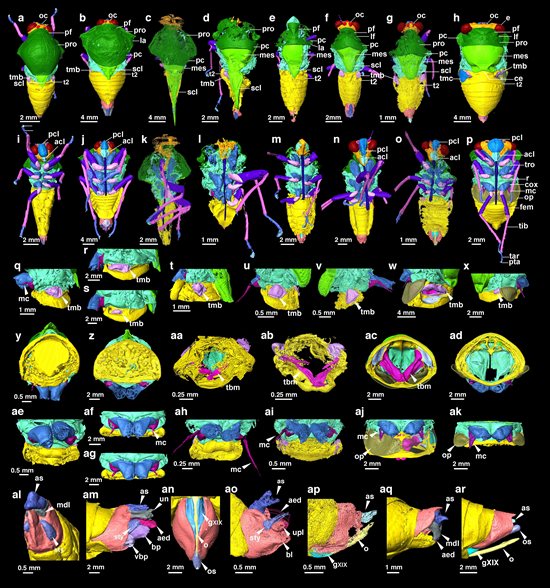
Digital reconstructions from micro-CT data reveal a diverse array of morphologies throughout the evolution of Cicadoidea.
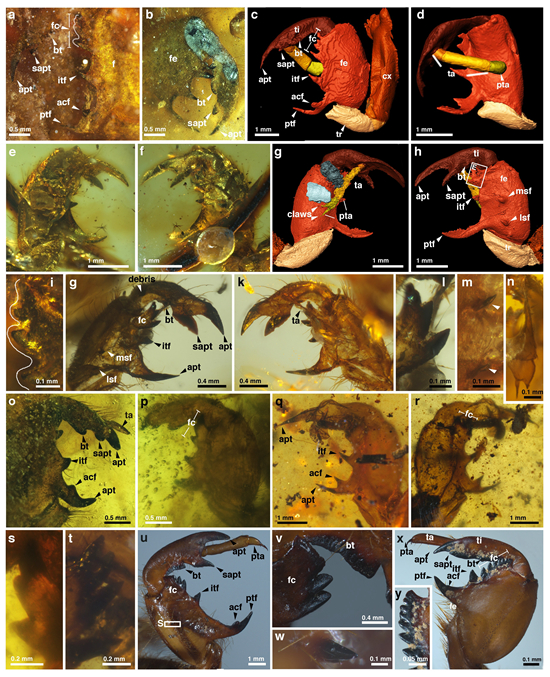
Specialized fossorial forelegs of fossil and extant cicada nymphs.
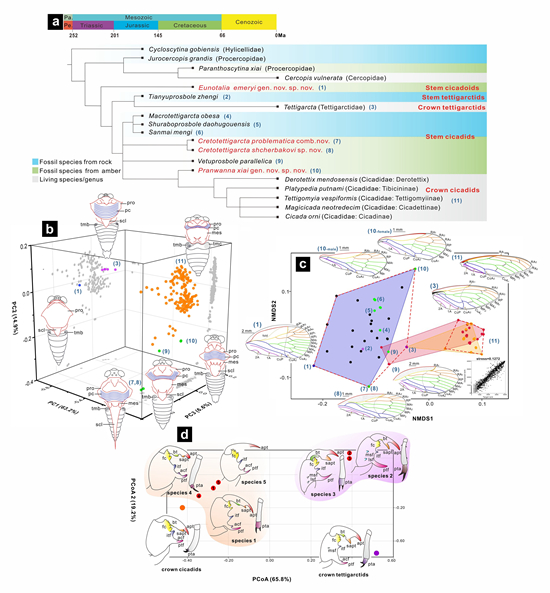
Phylogenetic and morphological analyses-based relative relationships discrimination and reconstruction.

Life reconstruction of cicadas in the Mesozoic forest.
Contact:
LIU Yun, Propagandist
Email: yunliu@nigpas.ac.cn
Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences
Nanjing, Jiangsu 210008, China
Download:
